With an increasing focus on Alzheimer’s disease research and treatment, the Alzheimer’s Association International Conference (AAIC), the world’s largest meeting dedicated to advancing dementia science, returns to Philadelphia this summer from July 28th-August 1st.
The annual event brings together researchers, clinicians, and dementia professionals to share ideas and showcase breakthrough research discoveries and improvements in the prevention, treatment, and diagnosis of Alzheimer’s disease.
See VUNO’s press release announcing 3 upcoming poster presentations at AAIC 2024.
Recap of the 2023 AAIC Conference
Last year’s conference took place in the Netherlands, bringing 10,000 attendees and more than 3,000 scientific presentations in-person and virtually. With registration still open for 2024, AAIC is now in the U.S.!
American professionals will have more opportunities to learn from their overseas counterparts in 2024: at the 2024 conference, 6 main speakers will be from outside the U.S., compared to 2 in 2023.
To help attendees prepare for AAIC 2024, the VUNO team gathered an overview of all upcoming sessions for consideration.
In-Person Workshops and Pre-Conferences at AAIC 2024
Before the start of the main conference, AAIC 2024 will offer in-person workshops and pre-conferences from July 25th to 27th. These will give attendees the opportunity to explore and educate themselves on breakthrough findings and new practices that advance the prevention and treatment of Alzheimer’s disease and other dementias.
The workshop, “Improving Public Health Action on Brain Health: A Public Health Center of Excellence Workshop on How to Effect Change,” will focus on modifiable risk factors that can improve brain health and review how public health can better educate people about and promote brain health.
The workshop is sponsored by the Public Health Center of Excellence on Dementia Risk Reduction at the Alzheimer’s Association, which is funded by the U.S. Centers for Disease Control and Prevention (CDC), and is a great learning opportunity for all attendees.
Click here to review the full program for workshops.
2024 Scientific Sessions
One of the main events at AAIC is the Scientific Sessions, which include Plenary Speaker and Perspectives sessions. These sessions feature podiums and poster presentations that focus on science and pathogenesis, biomarkers, clinical manifestations, drug development, public health, dementia care, and the latest Alzheimer’s research.

Dr. Cynthia Lemere’s Plenary Session “The Evolving Role of Complement in Alzheimer’s Disease” at AAIC 2023
Plenary Speaker Sessions
The Scientific Sessions also include the Plenary Speaker Sessions, featuring discussions on advancements in Alzheimer’s research and care. This year’s speakers include Edward B. Lee, Timothy Miller, and Reisa Sperling.
Dr. Lee will present the topic “Neuropathology in a Multidisciplinary Age” on the first day of the conference. Dr. Lee is a Professor of Pathology and Laboratory Medicine at the Perelman School of Medicine at the University of Pennsylvania and is the Co-Director of the university’s Institute on Aging, specializing in Neuropathology, Cancer and Immunobiology.
Another 2024 Plenary Speaker, Timothy Miller, will present “Antisense Oligonucleotide Therapeutics for Neurodegenerative Diseases.” Dr. Miller is an experienced Neurologist at Washington University School of Medicine in St. Louis, where he leads the Miller Laboratory and the ALS Center.
Reisa Sperling, a Neurologist at Harvard Medical School and Co-Principal Investigator of the Harvard Aging Brain Study in Boston. Her research focuses on the detection and treatment of Alzheimer’s disease and has led multiple Alzheimer’s clinical trials. She will present the session, “Preclinical Alzheimer’s Dementia.”
Perspectives Sessions
Perspectives Sessions provide expert reviews of recent dementia-related advances and plans for the future of Alzheimer’s care and treatment. Perspectives Sessions at AAIC 2024 will include:
- Translating AD biomarkers into clinical practice in an era of precision medicine
- Future perspectives on how to conduct clinical trials in Alzheimer’s disease: Where do we go from here?
- Dementia and the provision of care: diverse lived experiences across the Black/African diaspora
Industry leaders will discuss, review, and debate these topics to advance Alzheimer’s care and treatment.
View full information on the Scientific Sessions here.
Emerging Concepts in Basic Science
The Emerging Concepts in Basic Science focuses specifically on dementia science. This series will explore three concepts:
1. The Causes and Consequences of Tau Aggregation in Tauopathies
Chaired by Bradley Hyman and Maria Grazia Spillantini, includes 4 sessions, such as Tau Post-Translational Modifications, presented by Judith Steen.
2. Immune Cells Diversity and Their Role in Cognition and Dementia
Chaired by David Holtzman and Diego Gomez-Nicola, features 4 sessions, including Diversity and Function of Brain Macrophages in Alzheimer’s Disease, presented by Kiavash Movahedi.
3. Spatial Biology in Neurodegenerative Proteinopathies: Uncovering Resilience vs. Vulnerability
Chaired by Bart DeStrooper and Lea T. Grinberg, 4 sessions will cover topics like Charting New Paths: The Role of Spatial Omics in Advancing Neurodegenerative Disease Research, presented by Rebecca Hodge.
Check out the full list of Emerging Concepts in Basic Science sessions here.
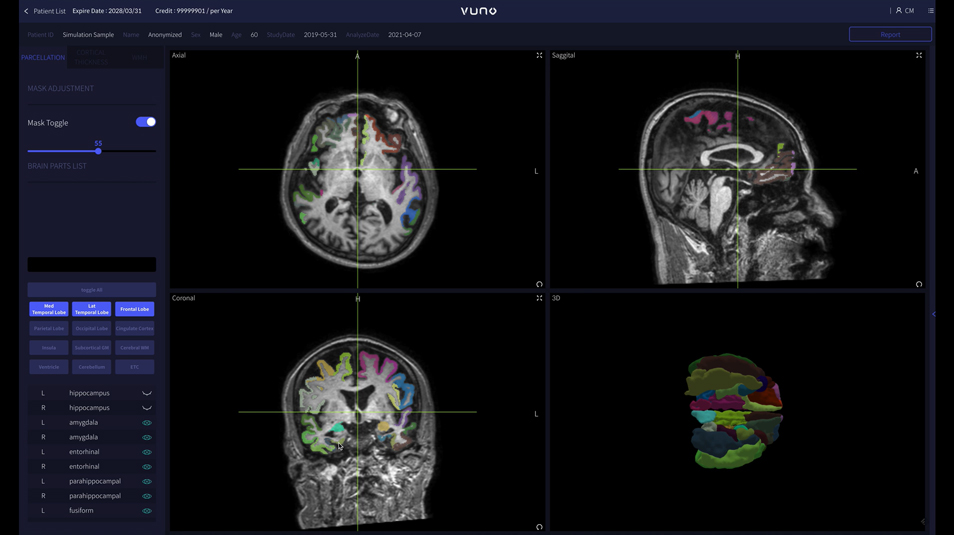
Visit booth #1411 to experience VUNO Med®-DeepBrain®

VUNO CEO Ye-ha Lee
This year, AAIC will host 117+ exhibitors leading the global medical and scientific dementia industry.
Find the full list of AAIC exhibitors here
Widely acclaimed as a true pioneer in AI-based medical solutions, VUNO will exhibit the FDA-cleared imaging solution VUNO Med®-DeepBrain® at booth #1411.
VUNO Med®-DeepBrain® a highly accurate and fast atrophy and WMH measurement AI solution to assist diagnosis of neurodegenerative diseases. the fastest, most accurate AI-based software for quantitative analysis of the brain on the market.
At AAIC 2023, VUNO had the honor of presenting a poster titled “Brain Volumetry-based Amyloid PET Positivity Prediction in Subjective Cognitive Decline Patients” during the pre-conference and conference sessions.
Interested in trying a demo of VUNO Med®-DeepBrain® before AAIC 2024? Book a Free Demo with our team. Connect with us for joint research projects or for any questions on AI assistants for imaging professionals.
Sources:
- Alzheimer’s Association International Conference (AAIC),
- AAIC 2023 at a Glance
- Plenary Speaker
- U.S. Centers for Disease Control and Prevention (CDC),
- Workshops and Pre-Conferences
- Scientific Sessions
- Perelman School of Medicine at the University of Pennsylvania
- Institute on Aging,
- Washington University School of Medicine in St. Louis
- Miller Laboratory
- ALS Center.
- Miller Laboratory
- Emerging Concepts in Basic Science
- Alzheimer’s Association